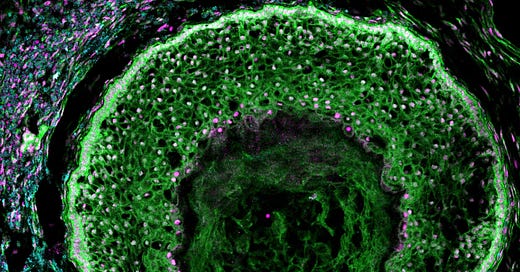
Concluding 2024: Weekly Highlights #24
ALSO: Legal Challenges of AI in Drug Discovery; Virtual Cells in Real Tissue; Disrupting In Vitro Fertilization Status Quo
Hi! This is
’s weekly newsletter, Where Tech Meets Bio, where we explore technologies, breakthroughs, and the great companies driving the biopharma and medtech industries forward.You might remember
covering the Aging Research and Drug Discovery Meeting in September’s article, From the Trenches of the 11th ARDD Meeting.Registration is now open for the …