Demis Hassabis on What's Next for AI in Biotech
ALSO: Adapting to Change in Clinical Development; AI is Key to Democratizing Drug Discovery; 10 Companies to Watch in Precision Medicine...
Hi! This is our weekly newsletter, ‘Where Tech Meets Bio,’ where we talk about technologies, breakthroughs, and great companies moving the biopharma and medtech industries forward.
If you've received it, then you either subscribed or someone forwarded it to you. If that is the case, subscribe by clicking this button:
BiopharmaTrend was invited to participate in Financial Times Live Global Pharma and Biotech Summit, a 3-day event co-organized by Financial Times and Endpoints News, and we gathered some valuable insights on strategic changes in clinical development, AI advancements, and partnerships in biotech. Here we would like to share some of them with you.
Let’s get to this week’s topics!
Brief Insights
🔬 World’s first stem-cell treatment for damaged corneas, developed by Kohji Nishida and colleagues at Osaka University in Japan, shows vision restoration in three out of four patients, with upcoming clinical trials planned to assess safety and efficacy further.
🔬 Elsevier has announced its support for the Pistoia Alliance through a program tackling AI challenges in drug discovery, focusing on data trustworthiness, transparency, governance, and skills development to address concerns around data quality and AI misinformation.
💰 Tempus AI announced plans to acquire genetic testing company Ambry Genetics for $600 million, expanding its capabilities in inherited cancer screening and other areas, including pediatrics, rare diseases, and immunology. Ambry will operate as a wholly owned subsidiary, with the acquisition expected to close in Q1 2025.
🔬 MIT researchers have created “wearable” devices for neurons—tiny, light-activated films that wrap around neuronal structures like axons without causing damage, enabling precise measurement and modulation of cellular activity. These azobenzene-based devices could someday aid in restoring brain function in conditions like multiple sclerosis by acting as synthetic myelin.
🔬 The UK government and Oxford Nanopore announce a partnership to launch a pandemic early warning system, expanding NHS England’s respiratory metagenomics program to quickly diagnose respiratory infections and enhance the UK's preparedness for future health threats.
🔬 Projected to save €5.5 billion over a decade, BC Platforms launches the EHDS-Ready Trusted Research Environment to help EU healthcare institutions comply with upcoming European Health Data Space regulations, enabling secure, cross-border data sharing for research and policy.
🔬 BC Platforms, Modirum Platforms, VEIL.AI, and Productivity Leap have introduced an AI-enhanced architecture for implementing the European Health Data Space secondary (EHDS2) framework, automating GDPR-compliant data management to enable secure, cross-border research and streamlined data requests across EU healthcare institutions.
💰 According to the British Business Bank report, UK life sciences VC funds are outperforming the general market with a DPI multiple of 1.14, showing robust returns despite recent valuation declines. UK VC remains competitive with US and European markets, and fund managers express optimism about improved exit opportunities amid fundraising challenges.
💰 Research Grid raised $6.48M in seed funding, led by Fuel Ventures, to automate clinical trial administration using proprietary AI technology, focusing on patient recruitment, data management, and reporting to reduce delays, with projected 45% cost savings and a 145% increase in patient engagement.
🔬 Tango Therapeutics is halting its TNG908 trial for glioblastoma due to low efficacy and shifting focus to two other PRMT5 inhibitors, TNG462 and TNG456, which show greater promise in clinical trials, particularly for CNS cancers and MTAP-deleted tumors.
🔬 BPGbio presented data at SITC 2024 on two oncology candidates, BPM31510 and BRG399, leveraging AI to develop immune-modulating therapies for glioblastoma and other "immunologically cold" tumors.
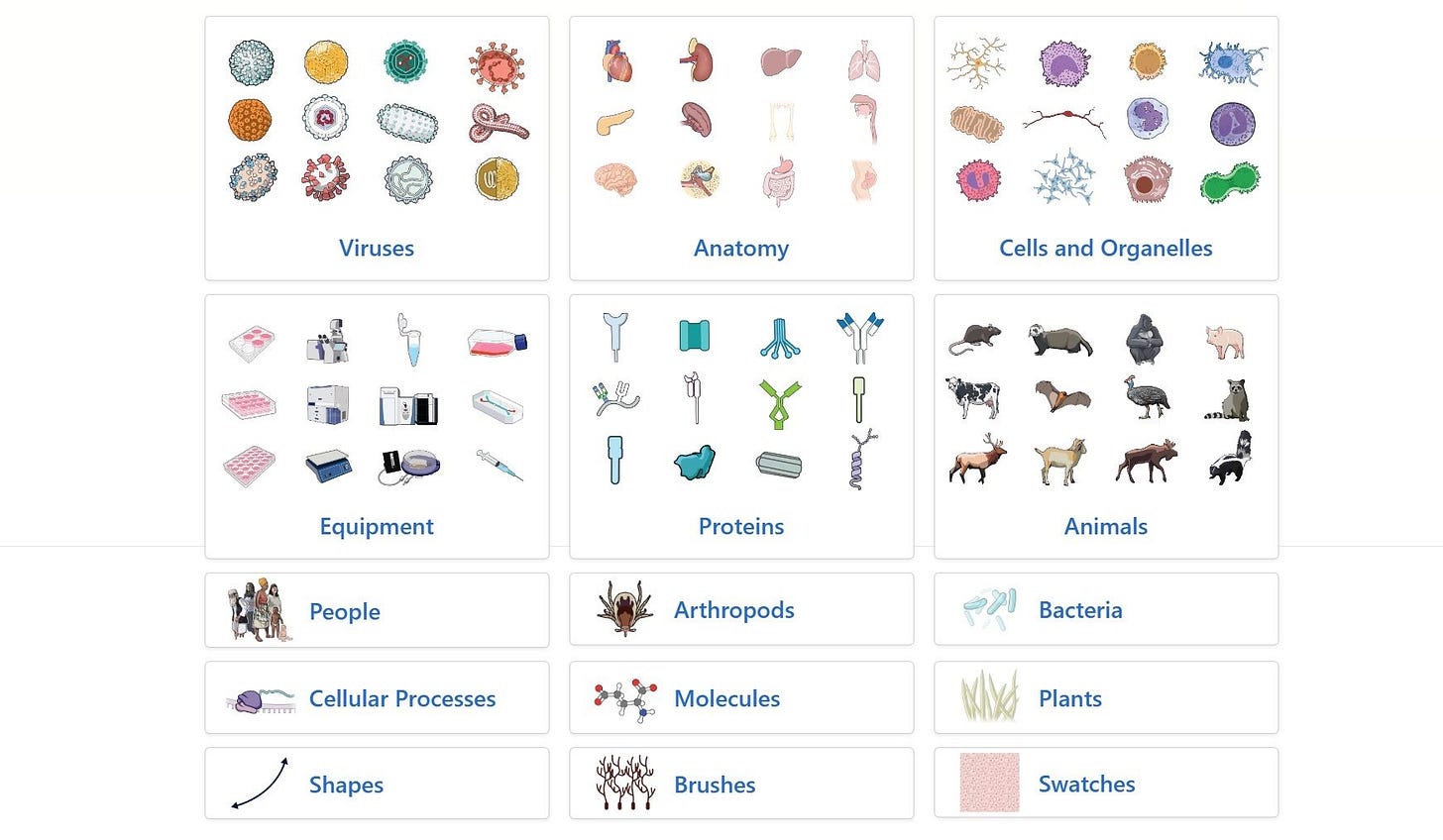
Demis Hassabis on What's Next for AI in Biotech
On the second day of FT Live, Demis Hassabis—CEO of Google DeepMind and Isomorphic Labs and co-creator of the Nobel Prize-winning AlphaFold—shared his vision for the future of AI in drug discovery.
Here’s what’s next, according to Hassabis:
AlphaFold2 marked a turning point in predicting protein structures, but AlphaFold3 steps up the game by tackling complex molecular dynamics, including RNA, DNA, and ligand interactions. Hassabis envisions an ambitious goal—a “virtual cell” model, allowing simulations of cellular processes to aid drug discovery. Realistic ETA? Around a decade.
Unlike many biotechs, Isomorphic Labs has opted not to build its own wet labs. Instead, Hassabis and his team are laser-focused on maximizing data efficiency and algorithmic innovation. The lab-in-the-loop concept stands out, where existing data is pushed to new limits with synthetic data, advancing AI model sophistication without the usual lab setup.
Developing accurate, AI-driven toxicity and safety predictions remains challenging due to limited historical data. However, Isomorphic Labs is actively exploring ways to “fill in the gaps” with generated and bespoke data, aiming to boost preclinical predictiveness.
With partnerships at Novartis and Eli Lilly, Isomorphic Labs gains valuable, experimental data access, which it combines with its AI-driven models. This partnership approach lets the company focus on tough-to-crack problems—e.g., “undruggable” targets.
Hassabis highlighted the essential balance between open science and biosecurity, especially as AI tools become more powerful and adaptable. The goal is to ensure responsible use without stifling innovation.
Exciting times ahead as AI continues to reshape biopharma. Hassabis’s “cautious optimism” serves as a reminder of the balance needed as these advancements progress.
Adapting to Change in Clinical Development
One of the FT Live panels "Adapting to Change in Clinical Development Through Strategic Partnerships" focused on how pharma and biotech companies are navigating complex market conditions, leveraging technology, and reshaping business models.
Some quick insights:
Capital constraints and reduced valuations are prompting startups to delay or scale back early-stage programs. This trend is pushing companies to focus on assets with near-term revenue potential, particularly in high-demand areas like oncology and rare diseases, where ROI is clearer and market needs are significant.
Personalized diagnostics are transforming patient stratification, especially in oncology and rare genetic disorders. Companies are investing in biomarker development and companion diagnostics to better identify patient subgroups, which reduces clinical trial costs, boosts regulatory approval chances, and enhances treatment efficacy through precision medicine.
Patient advocacy in rare diseases has led to increased use of FDA pathways like "Fast Track" and "Breakthrough Therapy" designations, allowing more frequent feedback and engagement with regulators. This approach not only shortens development timelines but also lowers regulatory risk, a vital strategy for areas with unmet needs, such as rare cancers.
Hybrid outsourcing models are replacing traditional full-service partnerships as pharma companies adopt a mix of traditional CRO relationships and functional service providers (FSPs) for specific tasks like data management, site monitoring, and patient recruitment. This blended approach is essential for containing costs in large, complex Phase 3 trials, which often exceed $100 million.
Increased diversity in clinical trials is expanding trial populations to underserved regions, a shift that enhances data accuracy but presents logistical challenges. Pharma companies are investing in decentralized trial technology and local infrastructure to access these populations, aiming to reduce bias and improve generalizability, though at an added cost in staffing and logistics.
AI tools are transforming trial design in fields like oncology and complex chronic diseases. Predictive models optimize patient recruitment, site selection, and dropout rate predictions, improving trial efficiency. For instance, in Phase 2 trials, AI-driven models now help refine protocols and predict success likelihood based on historical data.
Regulatory shifts, particularly the Inflation Reduction Act’s provision for reimbursement caps based on initial indications, have influenced strategic focus. Companies are increasingly investing in "blockbuster" assets and avoiding drugs with multiple indications, as follow-on approvals might bring diminished returns. This change is reshaping portfolio strategies, especially for resource-limited companies that must prioritize assets with the highest financial impact.
AI is Key to Democratizing Drug Discovery
In a contributed article, CEO of OPTIC Andrey Doronichev shares his insights on how generative AI is transforming the life sciences industry.
Key takeaways:
Generative AI Accelerates Drug Discovery: Moving development into digital environments speeds up the process and cuts costs.
AI Enhances, Not Replaces, Scientists: AI empowers scientists to work more effectively, giving a competitive edge to those who adopt it.
Leveling the Playing Field: Advanced AI platforms make powerful tools accessible to smaller biotechs, democratizing drug discovery.
Expert Collaboration is Key: Combining tech and pharma expertise enables AI platforms to deliver precise, reliable results.
Strategic AI Partnerships Drive Success: Partnering with AI platforms is essential for biopharma companies aiming to lead in future drug development.
As quick bonus: the NIH just dropped a library of 500+ royalty-free scientific illustrations, a set of neat graphics to enhance presentations, posters, and articles. You can use those for your presentations, posters and articles.
10 Companies in Precision Medicine
This week, we’re highlighting a selection of companies in precision medicine—a field focused on tailoring treatments to patients based on genetic, environmental, and lifestyle factors to improve outcomes and reduce side effects.
As we usually do, we will be taking a glance at their technology.