12 Startups in the Digital Twin Healthcare Ecosystem: From Virtual Organs to Optimized Trials
How virtual replicas guide decisions in medicine, trials, and patient care.
Despite undeniable progress in life sciences over the last few decades, modern healthcare faces challenges on many fronts. Lengthy drug development processes, often spanning 10 to 15 years, suboptimal clinical trial designs that struggle with patient recruitment and retention, and a need for more personalised and preventive patient treatments contribute to inefficiencies.
The limited availability of physical resources and time constraints hinder real-time experimentation with new therapies, medical devices, and processes. Digital twin technology addresses these issues by enabling scientists and industry professionals to conduct experimentation in a virtual environment, streamlining processes and enhancing efficiency before introducing new inventions into the real world.
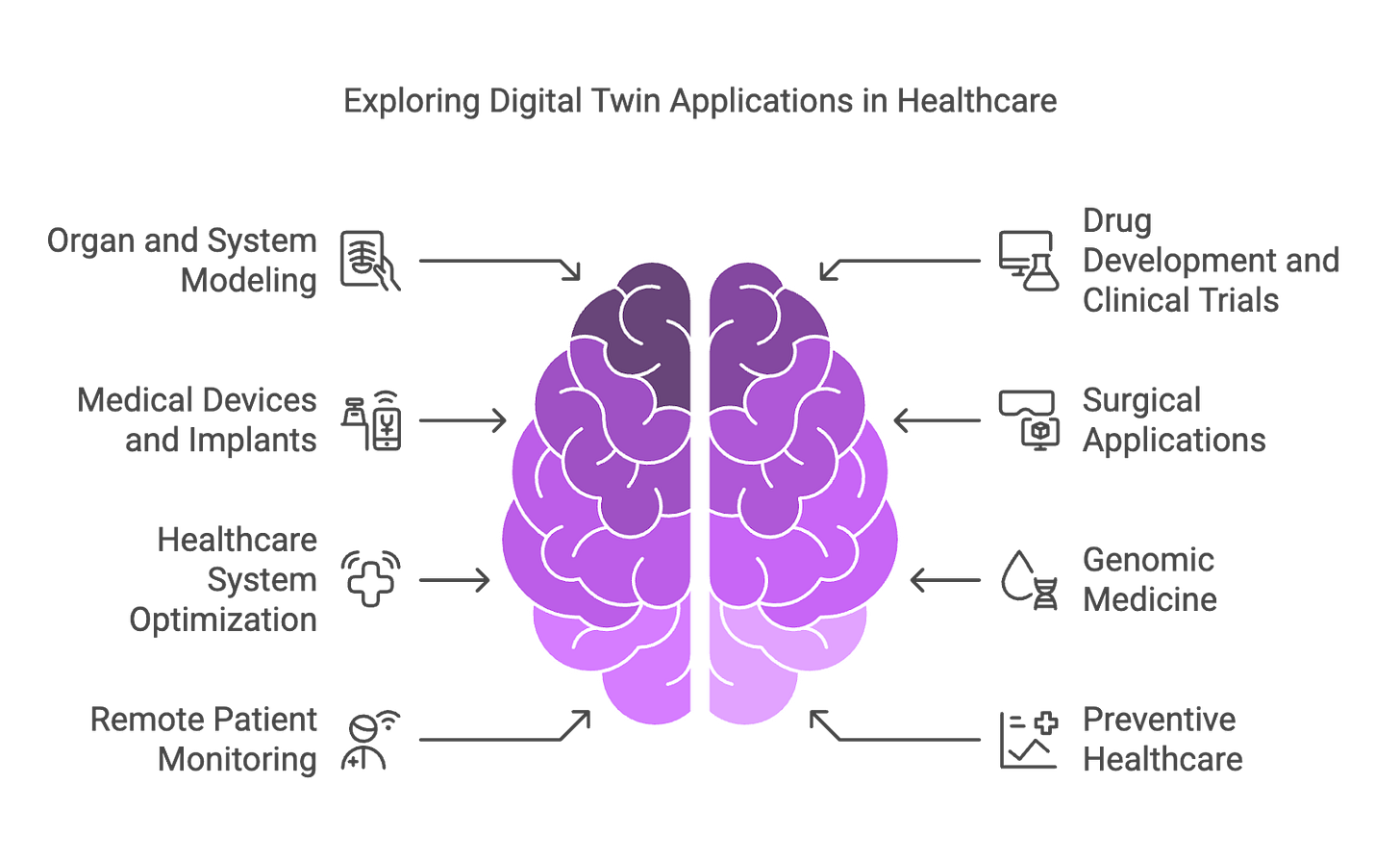
In this article: What Are Digital Twins? — Technology Overview — Biological Digital Twins — Experimental Digital Twins — Patient-Specific Digital Twins — Medical Device Twins
Cyprus has recently joined the group of 75 stakeholders supporting the European Commission's Virtual Human Twins (VHTs) Manifesto. The initiative aims to advance the development and integration of human digital twins within the European healthcare system while fostering global collaboration in the digital twin ecosystem. The signatories include companies, academic institutions, government bodies, computing centers, and healthcare providers from countries like the Netherlands, Belgium, Lithuania, Germany, Switzerland, and others across Europe and beyond.
The initiative receives €80 million in funding for research and innovation focused on computational models of patient pathophysiology, aimed at personalised disease management. An additional €24 million is designated for the development of an advanced digital platform for virtual human twin models under the Digital Europe Programme. The Manifesto highlights a new development in the healthcare digital twin domain, suggesting increased interest and potential for widespread application of this technology in future healthcare systems.
What Are Digital Twins?
Digital twins are dynamic virtual replicas of real-world physical objects, systems, or processes designed to optimise, simulate, predict, and analyse their real-life counterparts. Digital twin models are being increasingly applied in life sciences and clinical research, where the digital twin is a sophisticated virtual representation that mirrors the behavior of a physiological system, such as a human, an organ, or a cell. This technology enables real-time monitoring and optimisation of biological systems, providing insights and predictive capabilities for researchers and healthcare professionals.
The adoption of digital twins in life sciences has evolved over recent years, originally developed for industrial applications like manufacturing and engineering—digital twins have now expanded into healthcare and pharmaceutical domains, evolving together with the advancements in artificial intelligence (AI), Internet of Things (IoT), and big data analytics.
Technology Overview
In healthcare, digital twins consist of several key components that work in synergy. Internet of Things (IoT) sensors, devices detecting and measuring data about physiological and environmental parameters, and data collection mechanisms form the foundation, gathering real-time data from various sources such as wearables (smartwatches, glucose monitors, blood pressure monitors), medical equipment (insulin pumps, MRI machines, ECG devices), and electronic health records. These data are then processed using AI and machine learning (ML) algorithms, designed to enable more accurate predictions and personalised insights.
Cloud computing and big data analytics platforms provide the necessary computational power and storage capacity to handle the vast amounts of generated data, allowing for scalable and accessible digital twin solutions. Simulation and modeling software, enhanced by high-fidelity simulations and engineering-grade analyses, create dynamic virtual models that can replicate complex biomechanical behaviors and physiological processes.
Recent advancements in edge computing have considerably improved the real-time processing capabilities of digital twins, particularly in time-critical applications like tele-ICU systems. Edge computing processes data closer to the source—such as IoT devices, sensors, or local servers—reducing latency and enabling faster, localized decision-making, which is critical in healthcare settings where timely interventions can save lives.
Milestones driving these improvements include the integration of AI and ML algorithms directly into edge devices, allowing real-time analysis without sending data to the cloud. More powerful edge hardware has enabled complex computations locally, while the rollout of 5G networks has enhanced data transmission speeds. Enhanced security protocols also ensure sensitive patient data is processed locally with minimal risk of breaches.
🔹 For instance, San Francisco-based start-up Unlearn.AI develops AI-powered digital twins for clinical trials, employing ML models trained on historical patient data. Their platform generates synthetic control arms by creating virtual patient twins that simulate disease progression and treatment responses, reducing reliance on placebo groups. The technology integrates genomic, clinical, and wearable data to optimise trial efficiency and accuracy. In a 2024 Phase 3 Alzheimer’s trial partnership, retrospective analysis by Unlearn demonstrated the potential to reduce control arm sizes by 33%. In 2024, Unlearn raised $50 million in a Series C.
In cardiovascular sciences, digital twin technology is emerging as a tool that can assist in personalising complex surgical procedures, helping healthcare professionals with the intricate and highly individualized nature of cardiac conditions:
🔹 Philips develops virtual heart models that assist surgeons in planning complex cardiologic procedures. Their HeartModel system generates 3D models of patients' left heart chambers from 2D ultrasound images, utilizing a pre-trained anatomical framework refined with patient-specific data to assess cardiac function and blood ejection efficiency. Its EP Navigator integrates 3D CT/MR imaging with real-time fluoroscopy to guide catheter-based interventions for arrhythmias and structural heart disease. In 2024, Philips integrated AI into its cardiovascular ultrasound platforms, automating measurements like regional wall motion abnormalities and streamlining workflows. Its current innovations include MyoStrain AI, stemming from a collaboration with Myocardial Solutions, which detects early heart failure across 48 heart segments in 10 minutes.
Biological Digital Twins
In life sciences, digital twins come in various forms, including biological, experimental, patient-specific, and medical device models. Biological digital twins simulate complex biological systems, from cellular processes to organ functions. These models integrate multi-omics data and mechanistic simulations to predict biological behaviors and responses to stimuli.
🔹 Companies like AIBODY are advancing this field with their subcellular-level human digital twin, "Luke", which simulates normal and abnormal physiology, treatment options, and patient outcomes. Their platform is integrated into web and AR systems to assist in clinical decision-making and medical training.
🔹 Quibim, a Valencia-based company specialising in imaging biomarkers for precision medicine, is developing organ- and lesion-level digital twins, such as QP-Brain, QP-Prostate, and QP-Liver, to monitor health, stratify patients, and improve drug development success rates. These AI-powered models analyze routine medical scans like MRI, CT, and PET to precisely characterise phenotypes and predict outcomes in oncology, neurology, and metabolic disorders. QP-Brain quantifies brain atrophy and lesions to aid in diagnosing central nervous system disorders, while QP-Liver evaluates tissue fat and iron levels for liver disease management. With €47.9 million raised in 2025 Series A, Quibim collaborates with biopharma companies like Merck KGaA to integrate these models into clinical workflows.