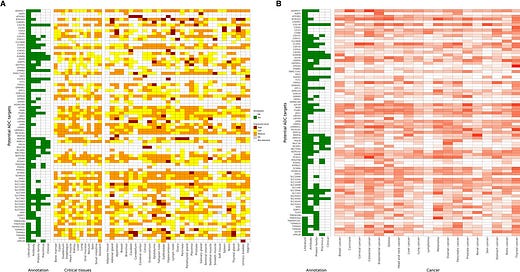
Lantern Pharma Introduces AI-Driven Antibody-Drug Conjugate Development
AI identifies 82 potential targets and 729 payloads for antibody-drug conjugates, including 22 already validated.
Lantern Pharma (NASDAQ: LTRN) has announced advancements in its RADR platform, integrating a new AI-powered module designed to accelerate and optimize the development of antibody-drug conjugates (ADCs). ADCs are targeted cancer therapies that combine antibodies specific to tumor cells with potent cytotoxic payloads. Reportedly, the global ADC market is …