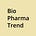Weekly Tech+Bio Highlights #27
Also: From LinkedIn to Drug Discovery; RNA Biomarkers for Predicting Better Responders; Recursion Expands into Clinical Trials, and More...
Hi! This is
’s weekly newsletter, Where Tech Meets Bio, where we explore technologies, breakthroughs, and the great companies driving the biopharma and medtech industries forward.If this newsletter is in your inbox, it’s because you subscribed or someone thought you might enjoy it. In either case, you can subscribe directly by clicking this button:
Let’s get to this week’s topics!
Brief Insights
🔬 Tempus AI launched olivia, an AI-powered health assistant app that consolidates records from EHRs, wearables, and manual uploads, offering AI-driven summaries and personalized care management for patients navigating complex healthcare systems.
🔬 Owkin enters the clinic with AI-powered cancer immunotherapy, dosing the first patient with a first-in-class EP2/EP4/DP1 triple inhibitor designed to counteract tumors’ immune suppression.
🚀 LinkedIn co-founder Reid Hoffman and cancer expert Dr. Siddhartha Mukherjee are teaming up to launch Manas AI, backed by $24.6M, to leverage AI and human expertise to accelerate drug discovery, starting with aggressive cancers like triple-negative breast cancer and lymphoma.
🔬 Lantern Pharma shines a light on antibody-drug conjugates as its new RADR platform module identifies 82 targets and 729 payloads, combining AI and massive datasets to cut ADC development costs by 60% and timelines by 30–50%.
🔬 Tubulis, fresh off one of Europe’s largest biotech fundraises, has dosed its first patient in a Phase I/IIa trial for solid tumors, advancing its ADC platform that recently attracted a licensing deal with Gilead.
🚀 Could a swarm of T cells reshape immunotherapy? Swarm Oncology emerged from stealth with its Swarm-T platform, designed to create vast numbers of potent, non-exhausted T cells to tackle the unmet challenges of solid tumors, as it prepares for clinical trials in 12–15 months.
💰 Evaxion Biotech raises $10.8M in IPO to advance AI-powered vaccine development, using its AI-Immunology platform to drive personalized cancer vaccines and infectious disease immunotherapies.
💰 Neomorph is zeroing in on oncology and immunology targets with molecular glue degraders, signing a $1.6B deal with AbbVie. This includes exploring therapies for hematologic malignancies and immune disorders by stabilizing interactions between disease-related proteins.
📈 Maze Therapeutics maps genetic solutions for kidney disease with plans for a $131M IPO to fund MZE829, an APOL1 inhibitor entering Phase 2 trials, and MZE782, targeting chronic kidney disease and phenylketonuria, while extending its cash runway into late 2027.
💰 Iceland’s rare genetic history drives innovation as Arctic Therapeutics raises €26.5M to advance AT-001, a Phase IIb/III drug targeting Hereditary Cystatin C Amyloid Angiopathy, a rare form of dementia linked to a mutation prevalent in Icelandic families.
💰 DNA’s overlooked regions may hold answers to cancer treatment—RyboDyn’s $4M pre-seed funding will expand its RyboCypher platform, which identified over 1,000 cancer-specific peptides from non-coding areas of the genome, unlocking previously “undruggable” targets for novel therapies.
🔬 Turning the slow gears of clinical trials into a streamlined machine, Advarra introduced its Study Collaboration solution, combining automation tools like milestone tracking and site training to eliminate common delays in trial startup timelines.
🔬 AI takes on one of cancer’s most complex pathways as Insilico Medicine doses the first patient with ISM6331, a pan-TEAD inhibitor targeting the Hippo signaling pathway in solid tumors, a notoriously difficult target now unlocked by Insilico’s Chemistry42 AI drug design platform.
💰 The precision of physics meets AI as Basetwo raises $11.5M to expand its hybrid Physics AI platform, allowing pharmaceutical engineers to simulate and optimize manufacturing processes in virtual settings before real-world implementation.
🔬 Trust in scientists remains strong—but public expectations are growing. A global study of 71,922 people across 68 countries found that 83% of respondents want scientists to actively engage with society, though concerns persist about scientists aligning with public priorities.
🔬 Guardant Health and ConcertAI partnered to create a data-as-a-service platform, combining 5.5M clinical records with genomic and epigenomic profiling to accelerate cancer therapy development.
📈 Aurion Biotech’s bid to restore vision moves forward despite legal hurdles, filing for a $100M IPO to expand Vyznova, its approved regenerative eye therapy in Japan, and advance AURN001, a next-gen candidate for corneal endothelial diseases now in Phase 1/2 trials.
🔬 Sarepta’s ELEVIDYS gene therapy advances Duchenne treatment amid ongoing controversy over its FDA approval process, which saw a top official override multiple review teams. The therapy has shown sustained benefits in trials, including improved mobility and muscle preservation over two years, offering hope for patients with this progressive disease.
📈 Suppressing hunger starts with bitter taste receptors—Aardvark Therapeutics plans an IPO to fund its lead drug ARD-101, which showed a 2.51-fold reduction in hunger in obese patients by activating gut-brain signaling, with potential for rare conditions like Prader-Willi syndrome.
🔬 In vivo gene editing offers a lifeline for beta-thalassemia patients, as YolTech begins clinical trials for YOLT-204, its lipid nanoparticle therapy designed to induce fetal hemoglobin production. Beta-thalassemia is a severe blood disorder requiring regular transfusions, and YOLT-204 could eliminate this dependence for patients.
💰 The “anti-CRO”—Lindus Health raises $55M to advance its AI-powered Citrus platform. Frustrated by traditional CROs' inefficiency and high costs, Lindus is building a tech-first approach to deliver faster and smarter trials across metabolic, respiratory, and women’s health diseases.
💰 The pursuit of fetal hemoglobin leads Novo Nordisk to IMMvention Therapeutix, licensing BACH1 inhibitors to boost its sickle cell disease pipeline after Forma’s acquisition, while IMMvention retains rights to brain-penetrant versions for potential neuro applications.
🔬 A new non-opioid painkiller lands FDA approval, as Vertex’s Journavx (suzetrigine), a sodium channel blocker, shows significant pain relief in surgical trials. Backed by fast-track designations, it marks a key opioid alternative for acute pain.
🔬 Algiax Pharmaceuticals’ small molecule achieves a Phase 2 win, showing “clinically meaningful” pain reduction in patients with peripheral post-surgical neuropathic pain.
🔬 Tris Pharma’s cebranopadol met its Phase 3 goal in post-surgery pain reduction, offering a non-addictive alternative to opioids as it gears up for FDA submission and chronic pain trials.
💰 Despite RNAi’s slow road to clinical success, Atalanta Therapeutics is betting on its brain-targeting platform, raising $97M to push RNAi therapies for Huntington’s and childhood epilepsy into Phase 1 trials, with additional programs in Parkinson’s and Alzheimer’s.
🔬 A psychedelic compound once sourced from toads—GH Research’s inhaled 5-MeO-DMT drug achieved rapid and sustained depression relief in a Phase 2 trial for treatment-resistant patients.
💰 AdvanCell raises $112M to advance its Pb-212-based targeted alpha therapy now in Phase 1/2 trials for metastatic prostate cancer. The funding will also expand manufacturing to secure isotope supply, a key hurdle in scaling radionuclide therapies.
🔬 Gameto’s Fertilo becomes the first iPSC-based therapy to enter a U.S. Phase 3 trial, aiming to transform fertility treatment by maturing eggs ex-vivo with lab-grown ovarian support cells. The trial follows the world’s first live birth conceived using this technology.
💰 Months after its IPO, BioAge drops lead obesity drug azelaprag due to liver-related side effects in a Phase II trial, shifting focus to its NLRP3 inhibitor pipeline
This newsletter reaches over 7.7K industry leaders from organizations like NVIDIA, Microsoft, Novo Nordisk, Pfizer, Novartis, FDA, the US Department of State, Illumina Ventures, and hundreds more.
Interested in sponsoring?
Contact us at info@biopharmatrend.com
From LinkedIn to Drug Discovery
Reid Hoffman, co-founder of LinkedIn, and Dr. Siddhartha Mukherjee, oncologist and author of The Emperor of All Maladies, have launched Manas AI (named after the Sanskrit word for “mind”), a new AI-driven biotech startup. Backed by $24.6 million in seed funding from General Catalyst, Greylock, and Hoffman himself, the company aims to combine artificial intelligence with wet-lab biology to accelerate drug discovery.
Manas AI is starting with aggressive cancers, including triple-negative breast cancer, prostate cancer, and lymphoma, with plans to expand to other diseases. It will use Microsoft’s Azure cloud computing platform to run its systems, capitalizing on Hoffman’s role on Microsoft’s board. Mukherjee will serve as CEO, leading the company’s efforts to integrate generative AI tools with human expertise to reduce drug development timelines from a decade to just a few years.
A Familiar Promise
This vision echoes a growing wave of AI drug discovery startups that have gained traction over the past few years. Despite significant investments in AI-driven drug discovery—$3.34 billion in 2024 alone—success stories remain sparse. Our report recently cited in Clinical Pharmacology & Therapeutics highlighted that eight leading AI companies collectively advanced 31 drugs into clinical trials. Of these, only a few reached Phase II, none achieved Phase III regulatory approval, and some were discontinued.
Dominika Wilczok and Dr. Alex Zhavoronkov, CEO and Co-founder of Insilico Medicine, outlined three primary business models in AI drug discovery:
Repurposing existing drugs: Identifying new disease targets for known molecules, often limited by efficacy challenges.
Designing new molecules for proven targets: Competing in crowded spaces where chemistry remains a bottleneck.
Designing novel molecules for novel targets: High-risk, high-reward platforms that demand rigorous validation but face substantial uncertainty.
Manas AI appears to fall into the third category, positioning itself as an end-to-end platform capable of identifying novel targets and designing first-in-class molecules.
See also: It’s Been a Decade of AI in the Drug Discovery Race. What’s Next?
Manas’ flagship initiative, Project Cosmos, aims to map the "rules" of how drugs bind to and interact with their biological targets. While ambitious, the project highlights recurring questions about what this startup can deliver that others have not.
AI-driven drug discovery continues to promise faster, cheaper pipelines, but the field has yet to consistently produce measurable success. Many companies offer little transparency regarding key metrics such as the number of molecules synthesized, timelines for progress, or success rates at different stages of development. Without clear, reproducible benchmarks, these promises remain difficult to validate, therefore the success of Manas AI—and the sector as a whole—will hinge on its ability to provide robust and transparent outcomes.
RNA-Based Biomarker Algorithms
Debiopharm and Genialis are extending their collaboration to develop predictive RNA biomarkers for WEE1-targeted cancer therapies. The goal is to refine patient selection and improve clinical outcomes by identifying those most likely to benefit from Debiopharm’s investigational WEE1 inhibitor, currently in Phase I trials.
WEE1 is part of the DNA damage response (DDR) pathway, which regulates how cancer cells repair themselves. Targeting DDR mechanisms has been a growing focus in oncology, with PARP inhibitors already approved for BRCA-mutated cancers (olaparib, talazoparib). Other DDR targets, including ATR, ATM, and WEE1, are in early clinical development but face challenges in balancing efficacy and toxicity. One key hurdle has been the lack of robust biomarkers to predict patient response—something this collaboration aims to address.
Genialis is using its Supermodel platform, an RNA foundation model (categorized as a large molecular model, or “LMM”), to analyze RNA sequencing data and classify patients based on biological signatures rather than relying on individual mutations or protein markers. The model was trained on hundreds of thousands of RNA-seq datasets to stratify patients into responder and non-responder groups while identifying potential combination therapies.
This collaboration follows a recent agreement between Genialis and Tempus AI, where Genialis will be using Tempus’ multimodal dataset to validate RNA-based biomarker models across multiple cancer types.
Not too long ago, Deep Genomics, a Toronto-based AI biotech, took a shot at leveraging RNA foundation models from another perspective.
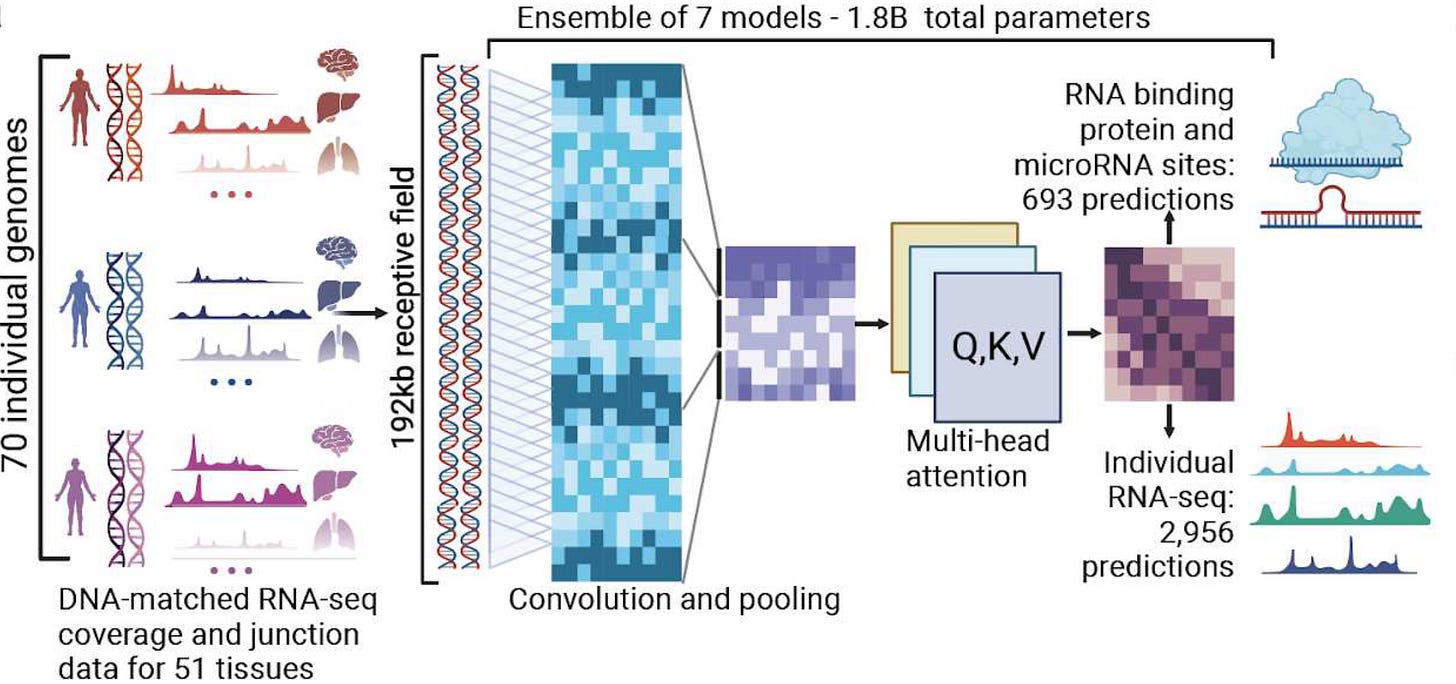
BigRNA is a foundation model trained on ‘one trillion genomic signals’ to uncover RNA disease mechanisms and design RNA-based therapeutics. The model is made to predict RNA-binding protein interactions, gene expression regulation, and splicing events, aiming to elucidate how genetic variants contribute to disease.
In June, 2024, we sat down with Brendan Frey, founder and CIO of Deep Genomics, to discuss how BigRNA was built, the challenges of applying foundation models to RNA biology, and what it takes to bridge the gap between AI predictions and real-world drug development.
While Genialis’ Supermodel focuses on biomarker discovery, BigRNA is aimed at drug discovery, identifying potential therapeutic targets and designing RNA-based treatments. Deep Genomics has already used its platform to generate drug candidates for conditions such as Wilson disease and spinal muscular atrophy.
Recursion Expands Into Clinical Tech
In a recent article in Genetic Engineering & Biotechnology News, Alex Philippidis interviewed Recursion’s Chief R&D Officer and CCO, Najat Khan, PhD, about the company’s ‘ClinTech’ initiative.
This new effort expands Recursion’s AI applications beyond drug discovery into clinical trials, targeting three key areas: optimizing trial design, accelerating patient enrollment, and enhancing evidence generation.
According to Najat Khan, clinical trials account for roughly 65–70% of the overall time and expenditure in drug development, compared with discovery. Recursion is investigating the use of real-world data and causal AI to simulate clinical trials before their initiation. This strategy is intended to help adjust patient inclusion and exclusion criteria, identify more suitable patient populations, and estimate the likelihood of operational success ahead of study launch.
The company is also exploring machine learning algorithms to pinpoint potential hotspots for locating eligible patients, an effort aimed at addressing the current challenge in which only about 4% of eligible patients enroll in clinical trials.
Recursion is also evaluating AI-driven methods for evidence generation. In open-label rare disease studies without a control group, the company plans to leverage AI to better contextualize patient natural history and bolster regulatory submission data packages.
This strategy is now being tested in Recursion’s clinical-phase oncology programs, including:
REC-3565, a Phase I MALT1 inhibitor being developed for multiple blood cancer indications. Unlike other MALT1 inhibitors, it avoids UGT1A1 inhibition, which could reduce the risk of hyperbilirubinemia.
REC-1245, a Phase I RBM39 degrader for biomarker-enhanced solid tumors and lymphoma, identified using the Recursion Operating System (OS). This program moved from target ID to IND-enabling studies in under 18 months, with only ~200 compounds synthesized.
REC-617, a Phase I/II CDK7 inhibitor for multiple advanced solid tumor indications. In December 2024, the program reported positive dose-escalation data, with one patient showing a confirmed partial response and a >30% reduction in tumor size.
The next milestone could be REC-4539, an LSD1 inhibitor for small-cell lung cancer, set to begin dosing in H1 2025. Recursion claims this is the first reversible and CNS-penetrant LSD1 inhibitor in development.
Adding to the news on AI-driven clinical trial optimization, now from academia—EPFL researchers are also developing AI-driven diagnostic tools to predict optimal treatments. Charlotte Bunne, head of the Artificial Intelligence in Molecular Medicine Group, emphasizes that biological data requires tailored AI architectures due to its multimodal and indirect nature.
Her team focuses on making AI models not just predictive but also interpretable, identifying biological mechanisms behind disease and treatment response. Their work extends to AI-powered Virtual Cells, an effort to build a multimodal, multi-scale foundation model simulating cellular behavior under different conditions.