Weekly Tech+Bio Highlights #35: Reading the Inflection Points of AI Drug Development
Also: Rice-Sized Dissolvable Pacemakers, Foundation Agents, & Industry Highlights
Hi! This is
’s weekly newsletter, Where Tech Meets Bio, where we explore technologies, breakthroughs, and cutting-edge companies.If this newsletter is in your inbox, it’s because you subscribed or someone thought you might enjoy it. In either case, you can subscribe directly by clicking this button:
Let’s get to this week’s topics!
🤖 AI x Bio
(AI applications in drug discovery, biotech, and healthcare)
🔹 AI-native biotech Xaira Therapeutics, which launched in 2024 with over $1B in funding, has appointed foundation model researcher Bo Wang to lead its biomedical AI efforts, expanding the company’s focus on integrating generative protein design and clinical optimization tools.
🔹 Researchers from 20+ institutions—including Stanford, Yale, DeepMind, Microsoft Research, and MetaGPT—have released a 264-page survey on the next generation of LLMs, introducing the concept of “Foundation Agents,” modular AI systems modeled after cognitive functions like memory, reasoning, and reward. — Dimitris Papadopoulos (Causaly) called it “a blueprint for scalable, adaptive systems” and the most comprehensive read on AI agents to date.
🔹 AI-driven biotech Plex Research is partnering with Ginkgo Bioworks to analyze large-scale transcriptomic data using its Plex AI platform, aiming to uncover new disease mechanisms and therapeutic applications by mapping how human cells respond to compound treatments.
🔹 Informatics company Revvity Signals Software has launched a unified data platform to streamline drug discovery workflows and enable AI-driven insights to help researchers explore complex and previously untreatable targets.
🔹 AI diagnostics company Viz.ai has received its third consecutive Edison Award for a machine learning tool that detects signs of hypertrophic cardiomyopathy based on ECG data across health systems.
🔹 AI company Unlearn has partnered with neurodegeneration-focused biotech remynd to apply digital twins in an early Alzheimer’s trial, using patient-specific simulations to help interpret biomarker changes in a small Phase 2a study. Unlearn also teamed up with Trace Neuroscience to guide clinical design in ALS.
See also: 12 Startups in the Digital Twin Healthcare Ecosystem
🔹 Parse Biosciences, the company that helped to build Vevo Therapeutics’ 100 million cell dataset for AI-powered drug discovery, has partnered with Japan-based SCRUM Inc. to expand access to its single cell sequencing platform across the country’s academic and biotech research communities.
🔹 Researchers introduce Med3DVLM, a vision-language model tailored for 3D medical imaging, enabling scalable multimodal reasoning across clinical tasks like retrieval, report generation, and VQA.
🔹 AI-driven biotech MultiOmic Health has partnered with Alloy Therapeutics to co-develop precision kidney-targeting drugs, combining patient stratification models with Alloy’s bispecific antibody and genetic medicine platforms.
🔹 Researchers have unveiled iKraph, allegedly the largest high-quality AI-driven biomedical knowledge graph to date, built from 34 million PubMed abstracts and 40 public databases. It maps over 10.7 million entities and 30.8 million relationships to support drug repurposing, biomarker discovery, and automated hypothesis generation.
🔹 Supply chain AI company ParkourSC will showcase real-world agent-driven use cases at LogiPharma 2025, joining Moderna, Zoetis, and SkyCell in a discussion on how imperfect data and systems still yield predictive power in pharma logistics.
🔹 Medtronic is teaming up with Barcelona-based Methinks AI to integrate AI-powered stroke triage software into its neurovascular portfolio.
🔹 Paige’s AI system for identifying cancer in multiple tissue types has received FDA Breakthrough Device designation, the first granted for a multi-tissue application in pathology, aimed at assisting pathologists in reviewing slides across a range of anatomical sites.
🔹 Researchers present MedAgentSim, a self-evolving open-source simulation where AI doctor agents engage in realistic clinical workflows—making diagnoses through multi-turn dialogue, test requests, and memory-based reflection—offering a dynamic benchmark for evaluating medical reasoning in large language models.
🔹 Berkeley and Caltech researchers release ProteinDT, an AI model that designs and edits proteins from natural language prompts, linking text to sequences via contrastive learning on 441K text-protein pairs, and outperforming prior models in zero-shot tasks.
🔹 Researchers led by Frank Noé and Cecilia Clementi have introduced BioEmu-1, a generative AI system that emulates protein equilibrium ensembles with up to 100,000x greater efficiency than traditional molecular dynamics simulations, while maintaining accuracy. The open-source tool predicts structures, folding free energies, and rare conformations from unseen sequences.
🔹 Genentech, Stanford, and a broader consortium of collaborators have introduced SpatialAgent, an AI system for spatial biology that autonomously handles experimental design, multimodal data analysis, and hypothesis generation.
🚜 Market Movers
(News from established pharma and tech giants)
🔹 Siemens is acquiring life sciences informatics firm Dotmatics for $5.1B, gaining control of widely used tools like GraphPad Prism and SnapGene along with the AI-native Luma platform that powers multimodal, low-code research automation for over 2 million scientists.
🔹 South Korea–based bispecific antibody biotech ABL Bio has licensed its blood-brain barrier shuttle platform to GSK for developing therapies targeting neurodegenerative diseases, in a deal worth over $2 billion.
🔹 Boehringer Ingelheim is investing $31M into a new Swiss research facility through its subsidiary NBE Therapeutics to advance the development of antibody-drug conjugates.
🔹 CellCentric, a clinical-stage biotech focused on cancer epigenetics, has opened a new office in Boston to support development of its oral therapy for multiple myeloma, expanding U.S. operations.
🔹 Precision oncology biotech Relay Therapeutics has laid off 70 employees in its third round of cuts within a year as it restructures to reduce R&D spending by 75%, aiming to prioritize a Phase 3 breast cancer trial and early-stage programs.
🔹 Bioavailability-focused CDMO AustinPx has launched a new lease program giving drug developers direct in-lab access to its formulation technology for small molecule drugs.
💰 Money Flows
(Funding rounds, IPOs, and M&A for startups and smaller companies)
🔹 A single-dose cell therapy showing seizure reductions—Neurona Therapeutics raised $102M to launch Phase 3 trial for a treatment targeting drug-resistant epilepsy.
🔹 RNA editing biotech AIRNA has raised $155M in Series B to fund Phase 1/2 trial for its lead therapy designed to correct the cause of alpha-1 antitrypsin deficiency, while also advancing RNA-based treatments for cardiometabolic diseases.
🔹 Immunology biotech RayThera has raised $110M in Series A to advance its small molecule drug candidates into Phase 1, backed by the same investor syndicate behind XinThera.
🔹 Investors continue to pour capital into AI-driven biotech despite uneven clinical results, with Isomorphic Labs recently raising $600M, and companies like Insilico, Recursion, Latent Labs, and Manas AI also drawing significant backing. — Meagan Parrish at BioPharma Dive.
🔹 Life sciences procurement platform Science Exchange has acquired HappiLabs, a specialist in lab purchasing support.
⚙️ Other Tech
(Innovations across quantum computing, BCIs, gene editing, and more)
🔹 Northwestern engineers have created a rice-sized, dissolvable pacemaker that can be activated by light through the chest, offering temporary heart support—especially for fragile newborns—without wires, batteries, or surgical removal.
🔹 Medivis has received FDA clearance for its AR- and AI-powered spine surgery navigation platform, enabling surgeons to visualize holographic spinal anatomy in real time during open and minimally invasive procedures without relying on traditional monitors.
🔹 New imaging-free spatial transcriptomics method reconstructs gene expression maps without microscopy, using molecular diffusion and dimensionality reduction to scale across large tissue samples. — Michael Montalto, Amgen’s VP of Precision Medicine, called it a step-change innovation, likening it to early AI-driven advances in pathology.
🔹 Japan Tobacco and D-Wave report that a quantum-assisted AI system trained large language models to generate more drug-like molecules than classical approaches alone, marking what they claim is the first time annealing quantum computing outperformed classical methods in this context.
🔹 Researchers at the University of Michigan have developed a brain-inspired analog controller that steers robots using just 0.25% of the power required by digital systems, showcasing real-time navigation with a rolling robot via memristor circuits that mimic neurons and perform computation directly where data is stored.
🚀 A New Kid on the Block
(Emerging startups with a focus on technology)
🔹 Differential Bio, a Munich-based startup combining AI, robotics, and microbiology to virtualize biomanufacturing scale-up, has launched from stealth with €2M in funding, aiming to cut long timelines and costs by simulating and automating microbial process development.
This newsletter reaches over 8.3K industry professionals from leading organizations across the globe. Interested in sponsoring?
Contact us at info@biopharmatrend.com
Is AI Drug Development Entering a Maturity Phase?
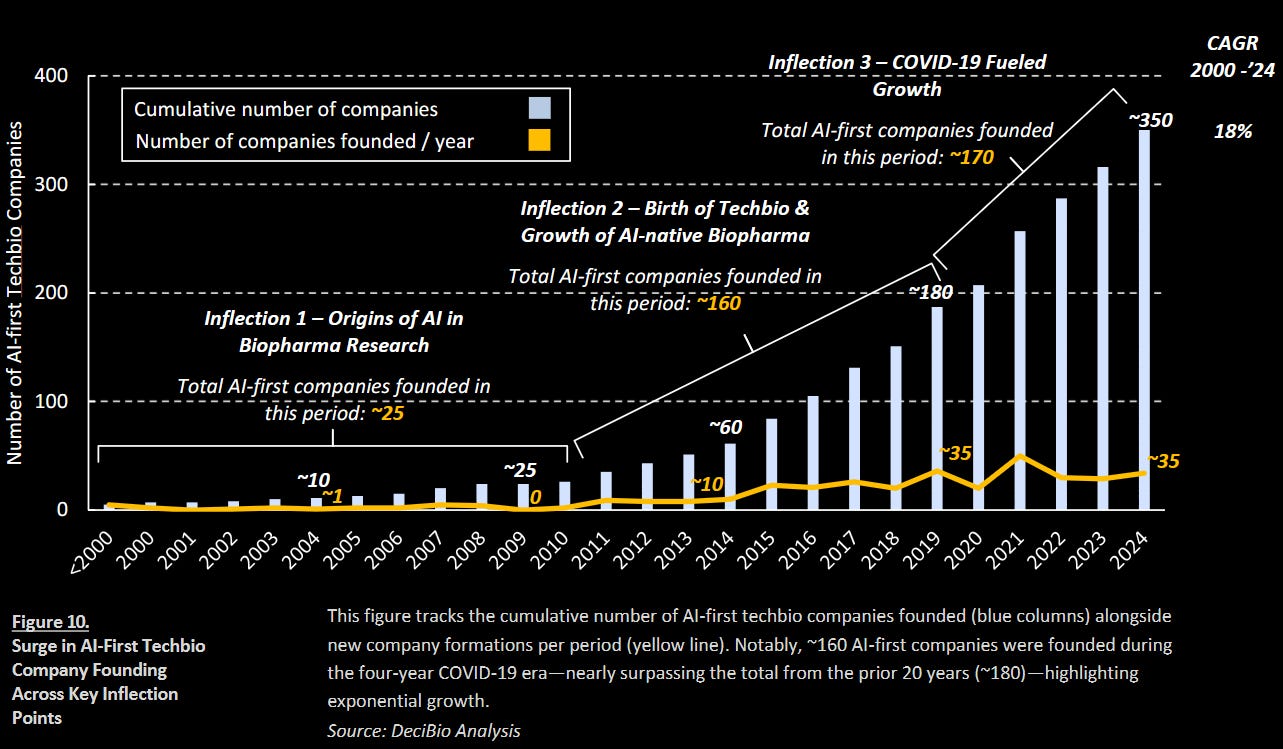
DeciBio’s new white paper, Inflection Points of Artificial Intelligence in Drug Development, outlines how AI’s role in drug R&D has evolved over the past three decades, culminating in what the authors describe as a new phase of domain specialization and maturity. Drawing on proprietary datasets, expert interviews, and pipeline analyses, the paper charts four inflection points: early rule-based systems, the emergence of AI-native biotechs, COVID-era acceleration, and the current return to innovation through multimodal foundational models.
According to the authors, one of the clearest signals of change is the volume of new entrants. Roughly 160 AI-first companies were founded between 2020 and 2024—nearly matching the 180 companies founded during the previous 20 years. These techbio players have expanded beyond SaaS into full pipeline development, prompted by funding opportunities and a search for validation. Notably, 2024 set a new record with $5.6B in AI-driven drug development funding, including Xaira Therapeutics' $1B launch.
Partnerships between biopharma and AI players—deal volume grew at 27% annually from 2019–2024, with the average deal size climbing to ~$1.5B. Importantly, milestone payments—rather than upfronts—grew fastest, signaling a shift toward performance-based models. Meanwhile, big tech expanded its infrastructure footprint: Nvidia introduced BioNeMo and Evo1, while Microsoft and Amazon pushed deeper into life science-specific cloud tools.
Despite these shifts, the field is still reckoning with unmet expectations: no fully AI-designed therapeutic has yet been approved, and many AI-first company pipelines center on relatively low-novelty targets. Post-IPO valuation losses and organizational challenges have also prompted consolidation, as seen in Recursion’s $688M acquisition of Exscientia.
DeciBio's analysis points to renewed momentum, driven by the maturation of self-supervised models, improved wet lab integration, and regulatory shifts. A widening capability gap is now forming between organizations scaling serious internal AI infrastructure and those maintaining surface-level engagement. With foundational models and multimodal data strategies gaining traction, the next inflection point may involve rethinking not just individual steps—but the architecture—of drug development itself.
Recent Fortune article further illustrates the transition from promise to execution in AI drug development, centering on Recursion’s effort to industrialize drug discovery through scale. The company now reportedly runs over 2 million weekly experiments, generating high-resolution cellular data across 50 human cell types and more than a trillion lab-grown neurons—fuel for its AI models running on BioHive-2, a supercomputer built with Nvidia.
Recursion’s infrastructure reflects a belief that success will hinge less on one algorithm than on tightly integrated hardware, wet-lab automation, and proprietary data pipelines. Still, the field remains marked by setbacks: Recursion’s lead drug, first identified during academic research, showed only mixed efficacy in a recent Phase 2 trial. CEO Chris Gibson acknowledges the gap between vision and results, saying that “…we are fundamentally changing the way that one discovers and develops medicine… but we have a long way to go”.
Injectable Light-Powered Pacemaker That Dissolves After Use
On the medical devices front, Northwestern University engineers have developed a pacemaker measuring just 1.8 mm × 3.5 mm × 1 mm, making it small enough to be delivered via syringe. Designed for short-term cardiac pacing—particularly in infants recovering from congenital heart surgery—the device dissolves harmlessly in the body after use, removing the need for extraction surgery.
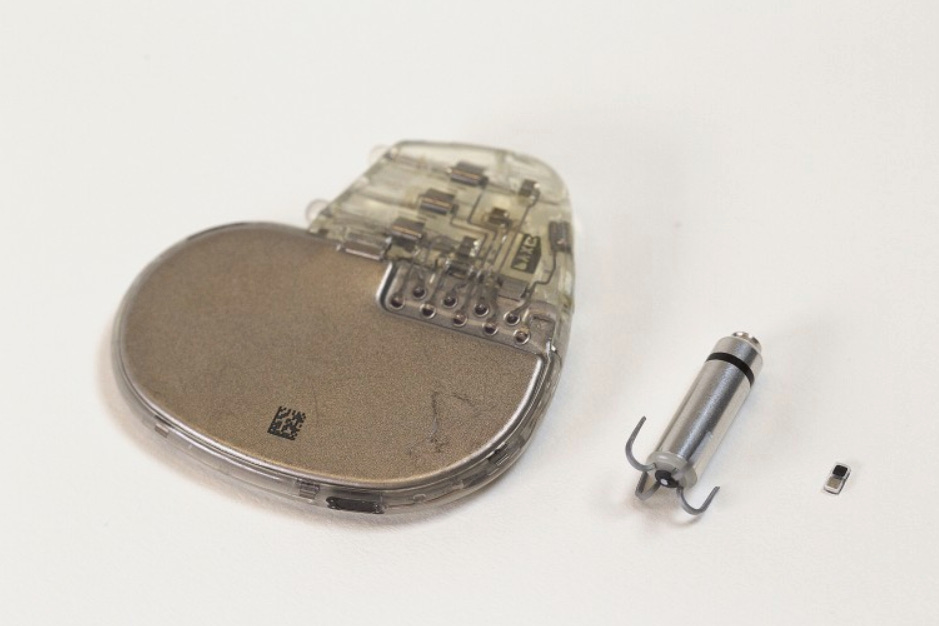
The system consists of two components: the bioresorbable pacemaker and a soft, wearable control patch that adheres to the patient’s chest. The patch uses infrared light to wirelessly activate the implanted device through skin, bone, and tissue, delivering synchronized electrical stimulation to the heart only when needed.
The device is designed to avoid traditional risks associated with temporary pacing wires, such as dislodgement, infection, and internal bleeding during removal. “Wires literally protrude from the body... When the pacemaker is no longer needed, a physician pulls it out”, said co-lead investigator Igor Efimov, “That’s actually how Neil Armstrong died”.
Unlike its predecessor, which relied on near-field communication and required a receiver antenna, the new version uses a galvanic cell powered by biofluids. This, along with light-based switching, enabled further miniaturization. Despite its size, the pacemaker delivers sufficient current for effective pacing and can be integrated with other implants, including heart valve replacements, to offer real-time rhythm control.
The device was validated in large and small animal models and in human donor hearts, as published in Nature. The project was led by bioelectronics researcher John A. Rogers and cardiologist Igor Efimov, in collaboration with teams at Dartmouth and the University of Chicago.