Where Are We Now With AI-driven Drug Discovery?🎄
The new research article raises important questions to focus on in 2025...
Hi! This is BiopharmaTrend’s weekly newsletter, ‘Where Tech Meets Bio,’ where we talk about technologies, breakthroughs, and great companies moving the biopharma and medtech industries forward.
If you've received it, then you either subscribed or someone forwarded it to you. If the latter is the case, subscribe by pressing this button:
First of all, Merry Christmas! 🎄
Now, let’s get to this week’s topic!
A new article "Progress, Pitfalls, and Impact of AI-Driven Clinical Trials" by Dominika Wilczok and Dr. Alex Zhavoronkov, CEO and Co-founder of Insilico Medicine, published in Clinical Pharmacology & Therapeutics, critically examines the impact of AI in drug discovery and development as of 2024.
What you should know:
Limited Clinical Success Despite Heavy Investment
While AI-driven drug discovery has seen billions in investment, few AI-discovered or designed drugs have entered human clinical trials, and none have achieved regulatory approval.
For example, a BioPharmaTrend.com report cited in the article highlights that eight leading AI drug discovery companies have collectively advanced 31 drugs into clinical trials, yet no AI-driven molecule has reached Phase III approval:
"According to a BiopharmaTrend report published in April 2024, eight leading AI drug discovery companies had 31 drugs in human clinical trials: 17 in Phase I (including one terminated), five in Phase I/II (including one discontinued), and nine in Phase II/III (including one with non-significant results).”
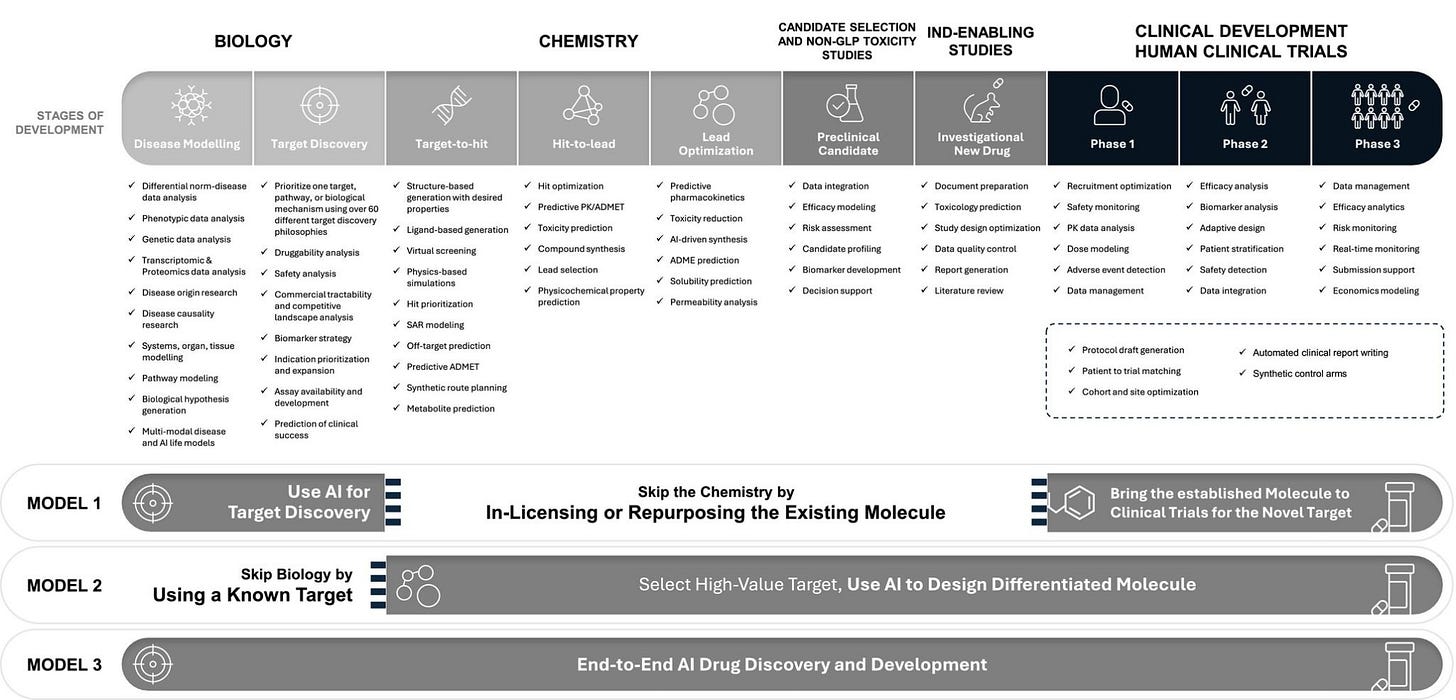
There are 3 Typical AI in DD Business Models:
First, repurposing known drugs or generics, where AI is used to identify disease targets and repurpose molecules for Phase II trials, but the approach often struggles with demonstrating efficacy.
Second, designing new molecules for established targets. Here, the focus is on best-in-class candidates with proven biology, this model faces intense competition and chemistry-related challenges.
Finally, some are designing novel molecules for novel targets. These are end-to-end AI platforms that identify first-in-class targets and design molecules, but require robust validation, as they operate at high levels of risk.
We need transparent industry Benchmarks:
For example, timelines for AI-discovered drugs have been reported to range from nine months to several years, but without consistent validation, these claims remain anecdotal.
AI still has limited impact in clinical development:
AI has automated processes such as medical writing and trial data analysis, improving efficiency but not significantly increasing drug approval rates. The real opportunity lies in integrating historical clinical and preclinical data to predict trial outcomes, identify optimal biomarkers, and inform trial design.
Validation and data challenges:
AI platforms often lack extensive validation in active drug programs, and disconnected public datasets limit the utility of AI models. Unified datasets and rigorous, real-world program validations are essential for scaling AI’s impact.
For those in AIDD, the message is clear: measurable, reproducible success is essential to fulfill AI’s promise in transforming drug discovery.
Read ‘Progress, Pitfalls, and Impact of AI-Driven Clinical Trials.’



I still don’t really understand how one defines “AI-driven drug discovery”. AI is a tool that will be adapted by nearly all companies in the space, and like any technology, some will leverage it more effectively than others.